恭喜实验室刘启阳(研究生)、刘轩麟(本)和王达(本)的文章被 J. Org. Chem. 接收发表
发布时间:2025-04-15 21:45:48
Visible-Light-Driven Tandem Cyclization of o-Hydroxyaryl Enaminones:
Access to 3-(a-Arylsulfonamido)trifluoroethyl Chromones
Jinwei Yuan,a,* Qiyang Liu,a Xuanlin Liu,a Da Wang,a Meng Yan,a,* Xianghui Meng,a Ji Ma,b,* Lingbo Quc,d
A visible-light-driven intermolecular tandem a-amidotrifluoroethylation/ cyclization of enaminones using a previously unreported N-trifluoroethylaminopyridinium salt was achieved in the absence of transition metal catalysts, or bases. Notable features of this synthetic method include mild conditions, high selectivity, excellent functional group compatibility, and satisfactory yields. Preliminary mechanistic studies indicate that the reaction proceeds via a radical pathway, involving an in situ generated N-trifluoroethyl radical followed by a 1,2-H shift.
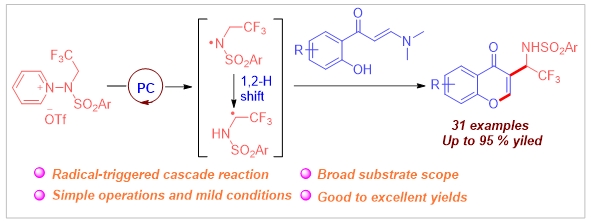
Access to 3-(a-Arylsulfonamido)trifluoroethyl Chromones
Jinwei Yuan,a,* Qiyang Liu,a Xuanlin Liu,a Da Wang,a Meng Yan,a,* Xianghui Meng,a Ji Ma,b,* Lingbo Quc,d
A visible-light-driven intermolecular tandem a-amidotrifluoroethylation/ cyclization of enaminones using a previously unreported N-trifluoroethylaminopyridinium salt was achieved in the absence of transition metal catalysts, or bases. Notable features of this synthetic method include mild conditions, high selectivity, excellent functional group compatibility, and satisfactory yields. Preliminary mechanistic studies indicate that the reaction proceeds via a radical pathway, involving an in situ generated N-trifluoroethyl radical followed by a 1,2-H shift.
