# 111 Visible-light-driven radical sulfonaminocyclization and sulfonylcyclization of acrylamides with N-sulfonaminopyrid
发布时间:2025-12-18 15:23:52
Visible-light-driven radical sulfonaminocyclization and sulfonylcyclization of acrylamides with N-sulfonaminopyridinium salts
Jin-Wei Yuan,a,* Wei Lian,a Yang Zhou,a Meng Yan,a,* Jian-Li Wu,b Liang-Ru Yang,a Yong-Mei Xiao,a Ling-Bo Quc,*
J. Catal. 2026, 454, 116625. http://doi.org/j.jcat.2025.116625
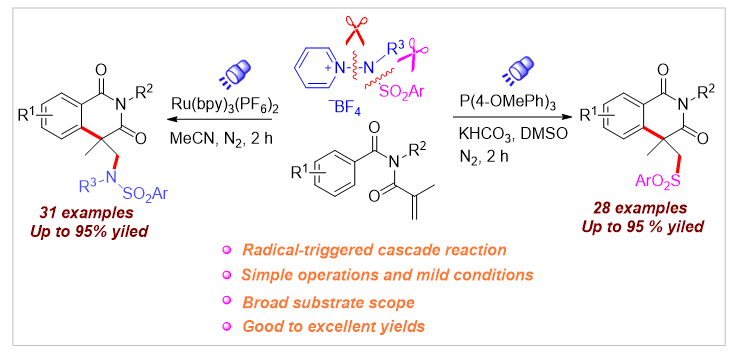
A photoredox-catalyzed sulfonamination/cyclization reaction of N-alkyl-N-methacryloyl benzamides has been developed, utilizing N-aminopyridinium salts as sulfonamino radical precursors via N-N bond cleavage. Additionally, this reaction also enables efficient N-S bond cleavage under mild conditions, generating diverse sulfonyl radicals through electron donor-acceptor (EDA) complexes. The innovative synthetic strategy provides a broad substrate scope and mild reaction conditions, facilitating the synthesis of various 4-sulfonamino and 4-sulfonyl isoquinolinonedione derivatives. Mechanistic studies suggest that the sulfonaminocyclization and sulfonylcyclization proceed through an electrophilic cyclization pathway, promoted by N-aminopyridinium salts with unactivated alkenes.
Jin-Wei Yuan,a,* Wei Lian,a Yang Zhou,a Meng Yan,a,* Jian-Li Wu,b Liang-Ru Yang,a Yong-Mei Xiao,a Ling-Bo Quc,*
J. Catal. 2026, 454, 116625. http://doi.org/j.jcat.2025.116625

A photoredox-catalyzed sulfonamination/cyclization reaction of N-alkyl-N-methacryloyl benzamides has been developed, utilizing N-aminopyridinium salts as sulfonamino radical precursors via N-N bond cleavage. Additionally, this reaction also enables efficient N-S bond cleavage under mild conditions, generating diverse sulfonyl radicals through electron donor-acceptor (EDA) complexes. The innovative synthetic strategy provides a broad substrate scope and mild reaction conditions, facilitating the synthesis of various 4-sulfonamino and 4-sulfonyl isoquinolinonedione derivatives. Mechanistic studies suggest that the sulfonaminocyclization and sulfonylcyclization proceed through an electrophilic cyclization pathway, promoted by N-aminopyridinium salts with unactivated alkenes.