# 118 Photoredox-Catalyzed Difluoromethylation/Cyclization of
发布时间:2025-12-18 15:01:35
Photoredox-Catalyzed Difluoromethylation/Cyclization of N-CyanamideAlkenes to Access Difluoromethylated Polycyclic Quinazolinones
Shilong Zhang,[a] Jinwei Yuan,*[a] Meng Yan,[a] ZhiyiWang,[b] Liangru Yang,[a] Yongmei Xiao,[a]
Shouren Zhang,*[c] and Lingbo Qu*[d]
Asian J. Org. Chem. 2025, 14, e00591. http://doi.org/10.1002/ajoc.202500591
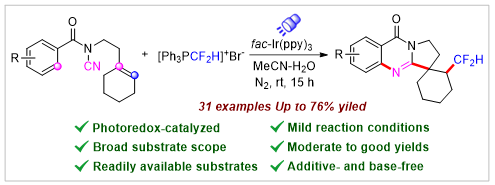
An efficient photoinduced radical cascade difluoromethylation/cyclization reaction of N-cyanamide alkenes has been developed. A diverse range of difluoromethylated quinazolinones have been synthesized with good yields and broad functional group tolerance. This photocatalytic protocol offers a facile and practical approach to accessing valuable polycyclic quinazolinone derivatives. Preliminary mechanistic studies suggest that the transformation proceeds via a radical pathway involving single electron transfer (SET).
Shilong Zhang,[a] Jinwei Yuan,*[a] Meng Yan,[a] ZhiyiWang,[b] Liangru Yang,[a] Yongmei Xiao,[a]
Shouren Zhang,*[c] and Lingbo Qu*[d]
Asian J. Org. Chem. 2025, 14, e00591. http://doi.org/10.1002/ajoc.202500591

An efficient photoinduced radical cascade difluoromethylation/cyclization reaction of N-cyanamide alkenes has been developed. A diverse range of difluoromethylated quinazolinones have been synthesized with good yields and broad functional group tolerance. This photocatalytic protocol offers a facile and practical approach to accessing valuable polycyclic quinazolinone derivatives. Preliminary mechanistic studies suggest that the transformation proceeds via a radical pathway involving single electron transfer (SET).