# 104 Photoredox-catalyzed tandem cyclization of enaminones with N-sulfonylaminopyridinium salts toward the synthesis of
发布时间:2024-01-11 12:14:45
Photoredox-catalyzed tandem cyclization of enaminones with N-sulfonylaminopyridinium salts toward the synthesis of 3-sulfonaminated chromones
Wenyu Hu,a Xiongqiong Diao,a Jinwei Yuan,a,* Wei Liang,a Wan Yang,a Liangru Yang,a Ji Ma,b,* Shouren Zhangc,*
The Journal of Organic Chemistry 2024, 89, 1, 644-655.
DOI: 10.1021/acs.joc.3c02399
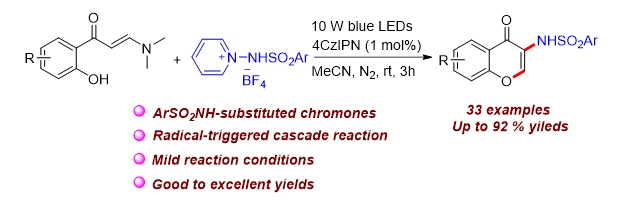
A photoredox-catalyzed intermolecular tandem sulfonamination/cyclization of enaminones was realized by using N-aminopyridinium salts as the sulfonaminated reagents without transition-metal catalysts, or bases. The reaction exhibits a broad scope and good functional group tolerance, good yields and regioselectivity. Preliminary mechanistic studies support the radical property of the reaction and the involvement of N-centered radical intermediates.
Wenyu Hu,a Xiongqiong Diao,a Jinwei Yuan,a,* Wei Liang,a Wan Yang,a Liangru Yang,a Ji Ma,b,* Shouren Zhangc,*
The Journal of Organic Chemistry 2024, 89, 1, 644-655.
DOI: 10.1021/acs.joc.3c02399

A photoredox-catalyzed intermolecular tandem sulfonamination/cyclization of enaminones was realized by using N-aminopyridinium salts as the sulfonaminated reagents without transition-metal catalysts, or bases. The reaction exhibits a broad scope and good functional group tolerance, good yields and regioselectivity. Preliminary mechanistic studies support the radical property of the reaction and the involvement of N-centered radical intermediates.